Our Discovery
Novel Patented Use Of Niacin FOR NASHFounders of Aasta Pharmaceuticals research team showed that niacin reduces and effects reversal of liver fat deposition (steatosis), inflammation and fibrosis in animal model for NAFLD and human hepatic stellate cells from NASH patients.
Effective & Unique Pharmacological Treatment
Niacin for NASH
In human primary hepatocytes, we have also showed robust inhibitory effects of niacin on oxidative stress and inflammation, major key players in the pathogenesis of NASH. The US Patent Office issued a patent for the new indication for the use of niacin, its metabolites and derivatives/analogs for the treatment and reversal of fatty liver disease.
Because niacin inhibits the earliest stage of the disease (fat deposition), it would be expected to also indirectly reduce the consequential incidence of NASH and fibrosis. Thus, niacin is uniquely suitable for combination therapy with drugs aimed at different targets to achieve enhanced synergistic efficacy.
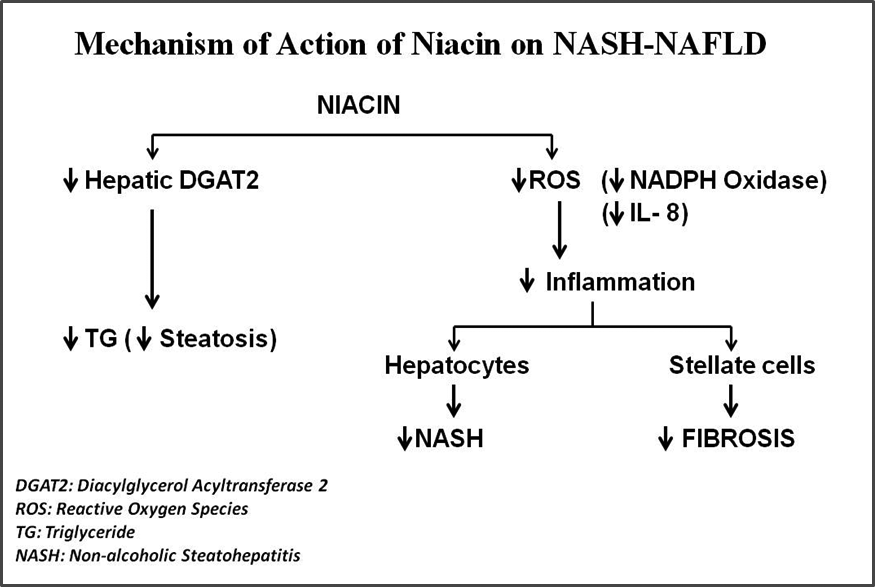
Mechanism of action of niacin
As illustrated in the schematic diagram, niacin acts on multiple critical targets in the pathological processes of NASH including:
I) Decreases hepatocyte triglyceride deposition by inhibiting diacylglycerol acyltransferase 2 (DGAT2)-a key and terminal enzyme in triglyceride synthesis. Thus, niacin markedly reduces development of liver steatosis arising from all three sources.
II) Decreases oxidative stress by reductions in Reactive Oxygen Species (ROS) and inflammatory cytokines resulting in decreased hepatocyte inflammation thus mitigating steatohepatitis (NASH)
III) In human stellate cells, niacin also reduces oxidative stress, inhibits and reverses collagen deposition thus mitigating fibrosis.
Clinical development of Niacin therapy for NASH
In pharmacologic doses, niacin is well known for lowering triglycerides and cholesterol. As monotherapy, it significantly reduced heart attacks and stroke similar magnitude to statins. In combination therapy with statin-based LDL-cholesterol lowering, it reversed coronary and peripheral vascular disease or delayed atherosclerosis progression. Thus, niacin-based therapy for fatty liver disease has the potential to benefit both conditions which often occur in the same patient.
Process
Clinical Development of Niacin Therapy for NASH
In pharmacologic doses, niacin is well known for lowering triglycerides and cholesterol. As monotherapy, it significantly reduced heart attacks and stroke similar magnitude to statins. In combination therapy with statin-based LDL-cholesterol lowering, it reversed coronary and peripheral vascular disease or delayed atherosclerosis progression. Thus, niacin-based therapy for fatty liver disease has the potential to benefit both conditions which often occur in the same patient.
Because crystalline niacin results in flushing (a nuisance but not a serious adverse effect) it was found that formulations that retarded its release rate decreased this side effect. However, the older very slow release (and no longer used preparations) resulted in hepatotoxicity. A unique preparation named Extended Release Niacin (ERN) generic for Niaspan was developed to mitigate the flushing and which was virtually free of hepatotoxicity and approved by the FDA. This generic formulation is available by prescription for treatment of dyslipidemia but not yet for NAFLD/NASH.
Aasta’s proposed development plan capitalizes on the vast amount of costly research done over 2 decades ago in the development of ERN. It is also important to note that niacin-based therapy for fatty liver disease has the potential to benefit both NASH and atherosclerotic cardiovascular disease (ASCVD).
The program aims to conduct following clinical trials aimed at producing one of the most effective therapies for NASH. Because niacin acts on a unique primary target (DGAT2) which controls the very initial biochemical step in liver triglyceride synthesis ( thus mitigating steatosis), combination formulation therapy with other drugs acting at another target , could result in a powerful synergistic combination drug. The other secondary target (oxidative stress) would also act to enhance efficacy.
Aasta is seeking partners who are interested in development of combinational drug development based on the rationale that such a combination will be superior to either drug alone. We hope to develop one of the best therapies aimed at treating NAFLD from inception to resolution.
Potential CLINICAL TRIALS
Phase 2a
The initial trial would be to assess the safety and dose of ER-Niacin in patients with NASH. It will be designed as a dose ranging (1000, 1500 and 2000 mg/daily) double-blind placebo-controlled randomized trial in patients with histologically defined NASH. This Phase 2a trial could be conducted in combination with partner’s Drug X in development
Phase 2b/3
Once the safety and dose of ER-Niacin is established, the next trial will be a Phase 2b trial in patients with NASH to assess the efficacy of ER-Niacin alone and in combination with drug X. Alternatively, a phase 3 trial could be conducted in discussion with regulatory authorities.
We invite partnership with companies who are interested in combination therapy to develop the most effective drug for NASH
Get In Touch
Moti Kashyap, MD
+1 310 721 8552
kashyapmoti@gmail.com
moti@aastapharma.com
Vijay Kamanna, PhD
+1 714 675 0561
vkamanna15@gmail.com
vijay@aastapharma.com
